
RNA Biology
RNA THERAPEUTICS
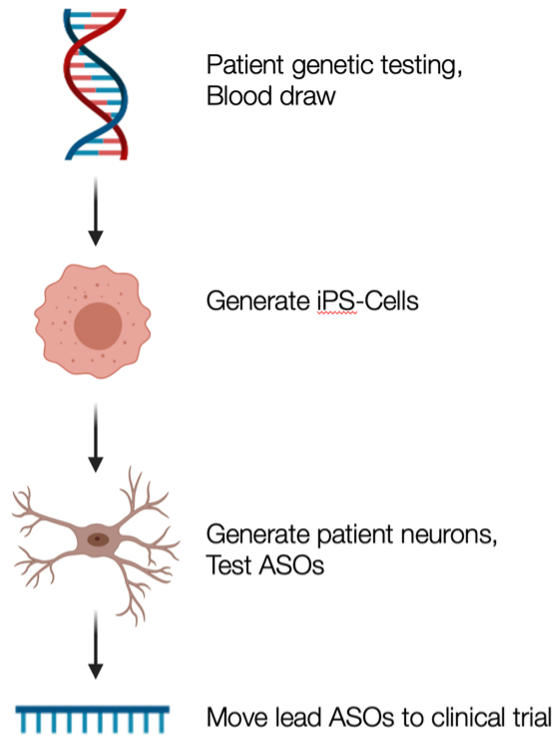
RNA is being increasingly harnessed for its therapeutic potential. While the most famous, widespread example is the mRNA vaccine developed against COVID-19, RNA therapeutics have also made huge strides in the last five years in combating rarer, genetic disorders. Antisense oligonucleotides (ASOs) are small, synthetic strands of RNA that can bind to a complementary region of a specific gene of interest and manipulate it in a multitude of useful ways.
Our team has developed novel antisense approaches to increase gene products that cause disease due to haploinsufficiency (loss of gene function). In one such approach, we use site-blocking ASOs to prevent microRNA-based repression of a target gene, “releasing the brake” on a particular gene of interest that is deficient in pathological states. In proof-of-concept studies, we show how disrupting microRNA repression of STXBP1 is capable of upregulating gene and protein expression to levels that are predicted to be therapeutic. We are currently pursuing this strategy for multiple leading causes of genetic neurodevelopment disorders due to haploinsufficiency.
RNA TRAFFICKING AND LOCAL TRANSLATION
If you’re a human over the age of 20, the same heart muscle cells currently beating in your chest will likely be with you until the end. Yet despite the fact that they live for decades, that cell has to completely turn over its inner contents every week or so, all without skipping a beat.
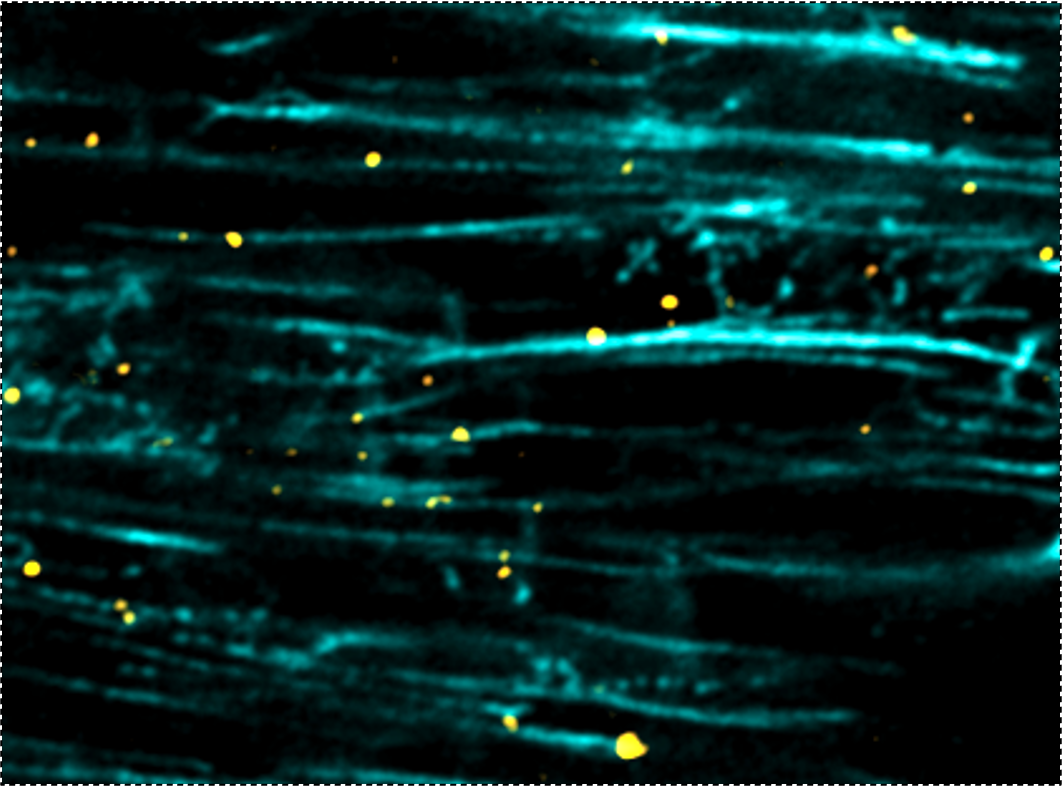
This requires exquisite control over where new proteins are made and how old ones are replaced. We recently discovered that motor proteins walking along microtubule tracks deliver multiple components of the protein synthesis machinery to the right place at the right time to locally control protein translation in the myocyte. When a heart muscle cell needs to grow (hypertrophy), this distribution system is ramped up to deliver the protein synthesis machinery to the sites of new cellular growth.
We aim to understand the molecular code that targets discrete RNAs or ribosomal components to specific subcellular locations, how this system is utilized to grow muscle cells in a specific direction, and how it may go awry in pathological states.
Related Publications
Mapping PTBP2 binding in human brain identifies SYNGAP1 as a target for therapeutic splice switching.
Dawicki-McKenna JM, Felix AJ, Waxman EA, Cheng C, Amado DA, Ranum PT, Bogush A, Dungan LV, Maguire JA, Gagne AL, Heller EA, French DL, Davidson BL, Prosser BL.
Nat Commun. 2023 May 6;14(1):2628. PMID: 37149717. doi: 10.1038/s41467-023-38273-3.
Cardiomyocyte Microtubules: Control of Mechanics, Transport, and Remodeling.
Uchida K, Scarborough EA, Prosser BL.
Annu Rev Physiol. 2022 Feb 10;84:257-283. doi: 10.1146/annurev-physiol-062421-040656. Epub 2021 Oct 6. PMID: 34614374. Review.
Microtubules orchestrate local translation to enable cardiac growth.
Scarborough EA, Uchida K, Vogel M, Erlitzki N, Iyer M, Phyo SA, Bogush A, Kehat I, Prosser BL.
Nat Commun. 2021 Mar 11;12(1):1547. PMID: 33707436. doi: 10.1038/s41467-021-21685-4.
Cardiac microtubules in health and heart disease.
Caporizzo MA, Chen CY, Prosser BL.
Exp Biol Med. 2019 Nov;244(15):1255-1272. Epub 2019 Aug 9. PMID: 31398994. doi: 10.1177/1535370219868960. Review.
